News Team member Justine Borgia explores strides in healthspan research geared to help humans live healthier for longer.
COVID-19 and Gene Expression: A New Understanding of Long-Term Impact
Discovering COVID-19’s role in altering gene expression and its long-term effects.
By Saif Hossain
Since the COVID-19 pandemic began in early 2020, scientists have struggled to grasp how an apparent respiratory virus created health problems that extend beyond the lungs and airways. The observed effects of COVID-19 on the circulatory system (in phenomena such as micro-clotting) warranted further study of how the virus affects the body’s inflammatory response. Now, a recent discovery at Weill Cornell Medicine shows that the virus can alter the gene expression of stem cells within bone marrow. These changes increase the risk of primed, excessive inflammatory responses that can persist long after the initial infection.
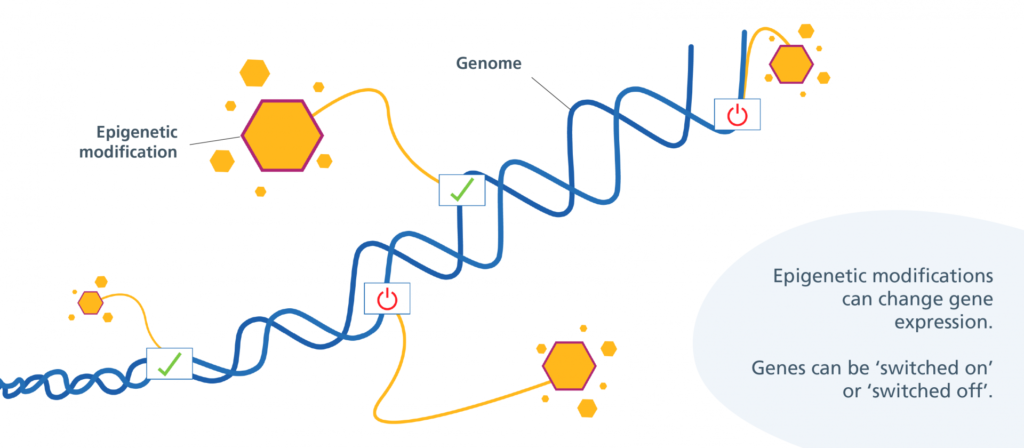
In August, Jin-Gyu Cheong and colleagues at Weill Cornell Medicine published a study in Cell detailing how patients with severe cases of COVID-19 experience epigenetic changes in the stem cells found in their bone marrow. This means that COVID-19 can cause bone marrow stem cells to turn certain genes on or off through the use of chemical markers. These markers change the shape of chromatin, the fibrous DNA-RNA-protein complex that makes up chromosomes. These stem cells can develop into various types of immune cells, and the researchers found that COVID-19’s modifications can last for up to a year after infection.
The team discovered that Interleukin-6, an inflammatory chemical produced by the immune system, plays a key role in stimulating these changes. The inflammation results in the production of underdeveloped white blood cells that do not contribute to an effective immune response. Worse, these cells are highly inflammatory and damage healthy tissues.
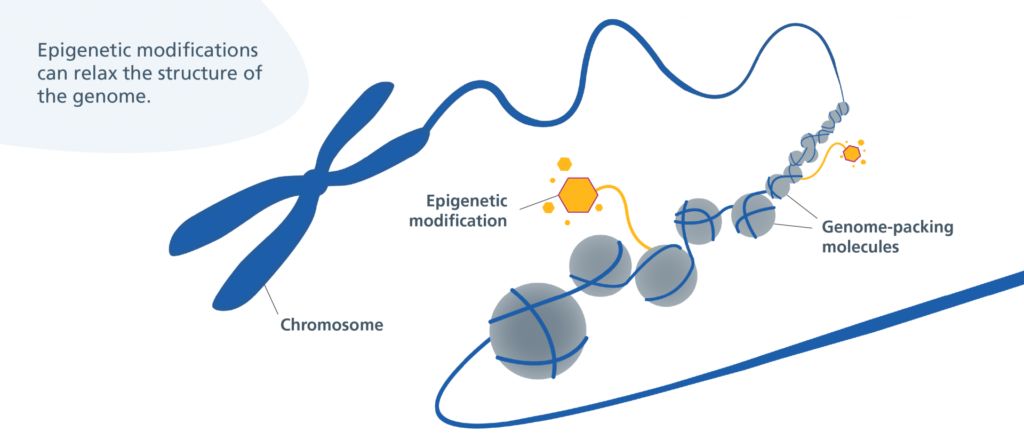
The unique nature of COVID-19’s inflammatory response
It’s normal for an infection to cause increased production of white blood cells; they help produce an efficient and rapid general immune response, which involves inflammation. They also help to protect against reinfection. What sets COVID-19 apart is its ability to cause prolonged, severe inflammation as part of the body’s immune response.
“[The stem cells] couldn’t remain blind to that level of inflammation for that period of time… And that was the trademark signature that we were looking for,” said Steven Josefowicz, associate professor at Weill Cornell Medicine and senior author of the Cell study. Josefowicz and his team specialize in researching the relationship between epigenetics, immune cell development, and how they respond to disease. He says COVID-19 can change gene expression in these bone marrow stem cells, creating a memory response within the innate immune system.
The immune system has two components: the innate and adaptive immune response. The innate immune system is responsible for the body’s general disease response and involves the recruitment of phagocytes (non-specific white blood cells), fever, and inflammation. Adaptive immunity, however, deals with specific recognition of antigens and encoding them in the memory system. Following exposure to disease, the body may prime itself against repeat infections by increasing its production of myeloid cells (a type of non-specific white blood cells), which results in trained immunity. Trained immunity is unique: it is a part of innate immunity but functions as a memory response.
“We don’t think of, classically, these innate cells…having memory, having the capacity to change,” says Dr. Rachel Sparks, a physician and clinical investigator at the National Institutes of Health. She says that the novelty of COVID-19 means that people don’t have pre-existing, cross-reactive antibodies, and it’s hypothesized that this can lead to a different type of immune response. “When you get an infection, we used to think your immune system went back to normal, right? But there’s increasing evidence that’s not the case.”
In the study, Josefowicz and his team of researchers at Weill Cornell Medicine obtained blood samples from patients recovering from severe COVID-19 infections. The team defined early samples as those collected from patients 2-4 months post-infection, while late samples were collected 4-12 months post-infection. These samples helped to visualize the chromatin within the patients’ chromosomes.
The researchers found that the chromatin in these patients’ bone marrow stem cells had different accessibility in both the early and late groups, compared to the controls. This indicates a difference in expression level. The stem cells favored the expression of more differentiated monocytes, a type of white blood cell that plays a major inflammatory role during an immune response. The observation of altered expression levels in both groups means that, for at least one year following severe COVID-19 infection, the stem cells in bone marrow produced hyperresponsive, inflammatory monocytes. The researchers also found that monocytes in both early and late groups were hyper-responsive against compounds that bind to specific receptors on cell membranes, indicating the presence of trained immunity.
Trained immunity and long-term health effects
The focus on trained immunity and its long-term health effects has increased in recent years. Induction of trained immunity has been used before to provide disease protection. For instance, bacillus Calmette-Guérin (BCG) vaccination conferred protection against the influenza A virus. However, it does not always work. Scientists experimented with BCG vaccination to try and protect against COVID-19 but to no avail. Other vaccines, such as the polio and flu vaccine, have shown limited success in inducing trained immunity against COVID-19. Results are preliminary, however, and further investigation is required to better grasp the mechanism of trained immunity.
Epigenetics was an overlooked part of COVID-19’s long-term ramifications. Trained immunity can protect against reinfection but may also produce adverse side effects. It likely contributes to long COVID. It may also contribute to chronic inflammatory diseases such as cardiovascular disease. Fortunately, Interleukin-6 blockers have shown promise against this harmful, uncontrolled inflammation. When the group studied late-group patients who received a blocker, they found decreased levels of monocytes and epigenetic differences with the rest of the late-group. They concluded that Interleukin-6 plays a definitive role in shaping the body’s trained immunity response to COVID-19. However, additional testing is needed to fully understand the effects of this inflammatory chemical and its chemical sources.
Trained immunity has both its advantages and its drawbacks. On one hand, it evolved to help protect against successive infections. On the other hand, it can contribute to harmful inflammatory processes. “These types of things occur on a spectrum,” said Josefowicz. “You have inflammatory disease; on the other side of the spectrum, you might have immunodeficiency. Your immune system might not be primed enough, and it could leave the individual susceptible to infection.”
