News Team member Stephanie Oehler describes how the online "trad-wife" aesthetic fuels the flames of the anti-vaccination movement during the second-largest measles outbreak of the 21st century.
A summary of important health news from the past week.
These Algorithms Could Bring an End to the World’s Deadliest Killer
By: Apoorva Mandavilli
With a quarter of all cases of Tuberculosis in India, the need for better access to diagnosis and early treatment is imperative. An Indian biotechnology company named Qure.ai has created an app with support from the Indian government called qXR. The A.I.-based app is able to analyze x-rays and can give a risk-rating of up to 27 various conditions, including Tuberculosis and Covid-19. In communities with very few doctors present and no specialists, this app has helped generalists doctors diagnose conditions earlier than before, and with greater certainty. The technology is expected to help decrease the overwhelming prevalence of Tuberculosis across the globe.
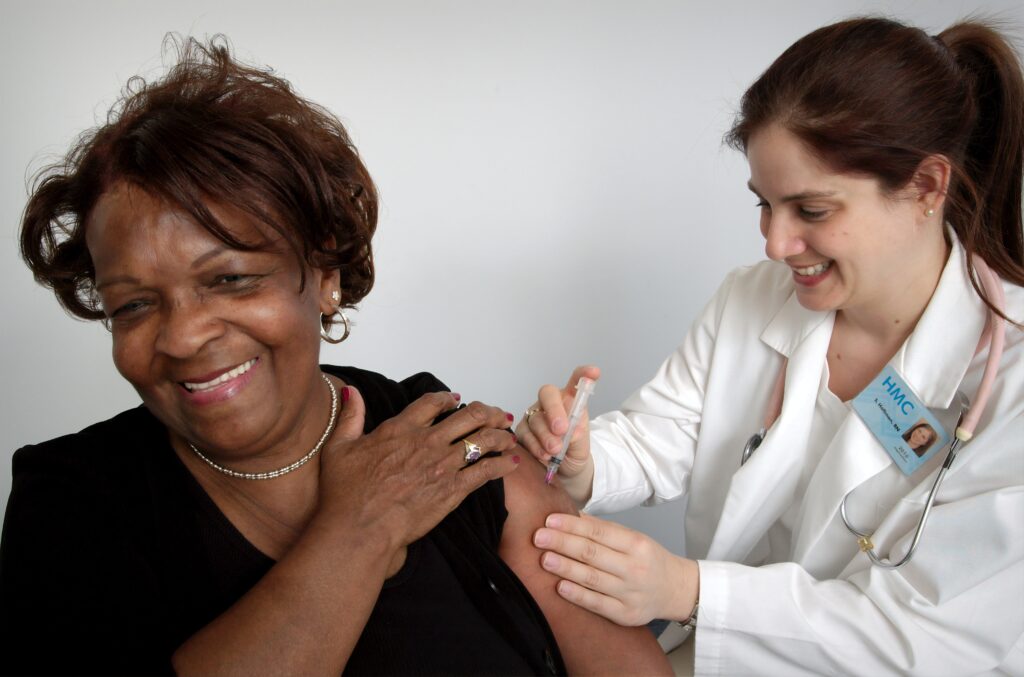
Covid-19 Vaccines: News and Updates
By: Vox Staff
Pharmaceutical companies are racing against the clock to develop a vaccine for Covid-19 first. New research is constantly being published online and it can be difficult to keep up with every update. Here is an update on major Covid-19 vaccine development from July to November.
Improve Emergency Care? Pandemic Helps Point the Way
By: Austin Frankt
When the pandemic began, health care administrators had to think quickly about their approach to delivering efficient and quality health care services. The practice of telemedicine rose in popularity as a result of the pandemic as a way to manage routine care while preventing the spread of the virus. For healthcare professionals, studies have shown that incorporating telemedicine into their daily medical practices could reduce wait times, patient mortality rates, and unnecessary healthcare costs. Telemedicine appointments would be given to The combination of telemedicine and regular in-person visits for the
Rapid At-Home COVID-19 Test Is Now Available
By: George Citroner
The first rapid at-home COVID-19 test is currently available for use. On Tuesday, the Food and Drug Administration (FDA) approved a new, rapid at-home COVID-19 test that must be prescribed to you by a healthcare provider first. It’s intended for people 14 years of age or older, but younger patients can use it as long as a medical professional performs the nasal swab for them. One cannot simply request one, however. To obtain a prescription, your doctor will have to suspect you’ve contracted SARS-CoV-2, the virus that causes COVID-19. According to Lucira Health, their all-in-one at-home diagnostic kit is a molecular test with analytical sensitivity, “or ability to detect the SARS-CoV-2 virus,” comparable to some of the best molecular tests “performed in clinical settings and high complexity labs.” Experts say the new test, while not perfect, could play a role in keeping businesses and schools open during the pandemic.
